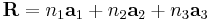Bravais lattice
In geometry and crystallography, a Bravais lattice, studied by Auguste Bravais (1850),[1] is an infinite array of discrete points generated by a set of discrete translation operations described by:
where ni are any integers and ai are known as the primitive vectors which lie in different directions and span the lattice. This discrete set of vectors must be closed under vector addition and subtraction. For any choice of position vector R, the lattice looks exactly the same.
A crystal is made up of a periodic arrangement of one or more atoms (the basis) repeated at each lattice point. Consequently, the crystal looks the same when viewed from any of the lattice points.
Two Bravais lattices are often considered equivalent if they have isomorphic symmetry groups. In this sense, there are 14 possible Bravais lattices in three-dimensional space. The 14 possible symmetry groups of Bravais lattices are 14 of the 230 space groups.
Contents |
Bravais lattices in at most 2 dimensions
In each of 0-dimensional and 1-dimensional space there is just one type of Bravais lattice.
In two dimensions, there are five Bravais lattices. They are oblique, rectangular, centered rectangular (rhombic), hexagonal, and square.[2]
Bravais lattices in 3 dimensions
The 14 Bravais lattices in 3 dimensions are arrived at by combining one of the seven lattice systems (or axial systems) with one of the lattice centerings. Each Bravais lattice refers to a distinct lattice type.
The lattice centerings are:
- Primitive centering (P): lattice points on the cell corners only.
- Body centered (I): one additional lattice point at the center of the cell.
- Face centered (F): one additional lattice point at center of each of the faces of the cell.
- Base centered (A, B or C): one additional lattice point at the center of each of one pair of the cell faces.
Not all combinations of the crystal systems and lattice centerings are needed to describe the possible lattices. There are in total 7 × 6 = 42 combinations, but it can be shown that several of these are in fact equivalent to each other. For example, the monoclinic I lattice can be described by a monoclinic C lattice by different choice of crystal axes. Similarly, all A- or B-centered lattices can be described either by a C- or P-centering. This reduces the number of combinations to 14 conventional Bravais lattices, shown in the table below.
| The 7 lattice systems | The 14 Bravais lattices | |||
| triclinic | P | |||
| monoclinic | P | C | ||
| orthorhombic | P | C | I | F |
| tetragonal | P | I | ||
| rhombohedral |
P | |||
| hexagonal | P | |||
| cubic |
P (pcc) | I (bcc) | F (fcc) | |
The volume of the unit cell can be calculated by evaluating a · b × c where a, b, and c are the lattice vectors. The volumes of the Bravais lattices are given below:
| Lattice system | Volume | |||
| Triclinic | 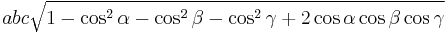 |
|||
| Monoclinic | 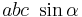 |
|||
| Orthorhombic | 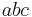 |
|||
| Tetragonal | 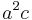 |
|||
| rhombohedral | 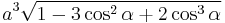 |
|||
| Hexagonal | 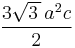 |
|||
| Cubic | 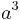 |
|||
Centred Unit Cells :
| Crystal System | Possible Variations | Axial Distances (edge lengths) | Axial Angles | Examples |
|---|---|---|---|---|
| Cubic | Primitive, Body centred, Face centred | a = b = c | α = β = γ = 90° | NaCl, Zinc Blende, Cu |
| Tetragonal | Primitive, Body centred | a = b ≠ c | α = β = γ = 90° | White tin, SnO2, TiO2, CaSO4 |
| Orthorhombic | Primitive, Body centred, Face centred, End centred | a ≠ b ≠ c | α = β = γ = 90° | Rhombic Sulphur, KNO3, BaSO4 |
| Hexagonal | Primitive | a = b ≠ c | α = β = 90°, γ = 120° | Graphite, ZnO, CdS |
| Rhombohedral (trigonal) | Primitive | a = b = c | α = β = γ ≠ 90° | Calcite (CaCO3, Cinnabar (HgS) |
| Monoclinic | Primitive, End centred | a ≠ b ≠ c | α = γ = 90°, β ≠ 90° | Monoclinic Sulphur, Na2SO4.10H2O |
| Triclinic | Primitive | a ≠ b ≠ c | α ≠ β ≠ γ ≠ 90° | K2Cr2O7, CuSO4.5H2O, H3BO3 |
Bravais lattices in 4 dimensions
In four dimensions, there are 64 Bravais lattices. Of these, 23 are primitive and 41 are centered. Ten Bravais lattices split into enantiomorphic pairs.[3]
See also
References
- ^ Aroyo, Mois I.; Ulrich Müller and Hans Wondratschek (2006). "Historical Introduction". International Tables for Crystallography (Springer) A1 (1.1): 2–5. doi:10.1107/97809553602060000537. http://it.iucr.org/A1a/ch1o1v0001/sec1o1o1/. Retrieved 2008-04-21.
- ^ Kittel, Charles (1996) [1953]. "Chapter 1". Introduction to Solid State Physics (Seventh ed.). New York: John Wiley & Sons. pp. 10. ISBN 0-471-11181-3. http://www.wiley.com/WileyCDA/WileyTitle/productCd-047141526X.html. Retrieved 2008-04-21.
- ^ Brown, Harold; Bülow, Rolf; Neubüser, Joachim; Wondratschek, Hans; Zassenhaus, Hans (1978), Crystallographic groups of four-dimensional space, New York: Wiley-Interscience [John Wiley & Sons], ISBN 978-0-471-03095-9, MR0484179
Further reading
- Bravais, A. (1850). "Mémoire sur les systèmes formés par les points distribués régulièrement sur un plan ou dans l'espace". J. Ecole Polytech. 19: 1–128 (English: Memoir 1, Crystallographic Society of America, 1949.)
- Hahn, Theo, ed (2002). International Tables for Crystallography, Volume A: Space Group Symmetry. A (5th ed.). Berlin, New York: Springer-Verlag. doi:10.1107/97809553602060000100. ISBN 978-0-7923-6590-7. http://it.iucr.org/A/
External links
- Smith, Walter Fox (2002). "The Bravais Lattices Song". http://www.haverford.edu/physics-astro/songs/bravais.htm